Summary Accrual is a simplified method in OnCore to report the total number of participants enrolled in a clinical research study, using periodic updates to track study accrual data. This method differs from typical OnCore use, which involves detailed data about individual participants and complete calendar information to log visit history and procedure coverage.
OnCore serves as an enterprise system and all clinical research investigators at UAHS are required to record and store their human subjects data in OnCore's database. In most cases, investigators must keep comprehensive information about their protocols, human subjects, and subject study visits, with timely data input (within 24 hours) after every research visit to ensure Banner Health billing compliance.
Summary Accrual can be used in special circumstances when OnCore's workflow features aren't needed for billing. Clinical research studies that do not involve Banner facilities or billable expenses, and that utilize REDCap for their subject management, may be considered for Summary Accrual, using minimum data (listed below) in OnCore. Meeting those two criteria does not necessarily mean a clinical research study can or should be considered for Summary Accrual. Additional considerations include cohort size and enrollment processes. Studies with large cohorts are more likely to be considered for Summary Accrual, especially if they are observational, retrospective, or banking studies.
The OnCore Support team will contact investigators whenever their initial review of a new protocol indicates that Summary Accrual may be advisable. Investigators are welcome to contact OnCore Support to request a review of their clinical research study to see if it qualifies for the Summary Accrual reporting process. Not all requests for Summary Accrual accommodation may be granted.
Accrual information can be provided monthly by an Excel spreadsheet (which will be uploaded into OnCore by the COM IT team) or updated directly in OnCore.
It is the research team’s responsibility to maintain and keep up-to-date information in OnCore, such as protocol status, staff, and IRB documentation.
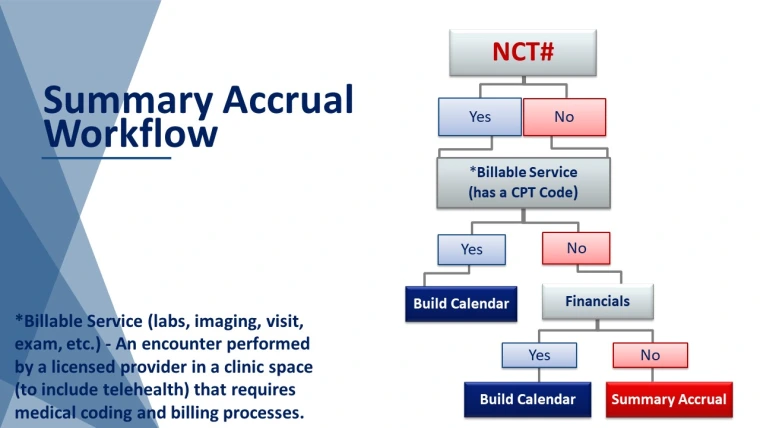
- The study does not have clinical services occurring in a Banner Health facility (e.g. blood draws, labs, EKG’s, etc.).
- The study will not use an OnCore calendar.
- Sponsor invoicing is not dependent on participant visit data.
- UAHS Research Administration must verify that the study qualifies. Once verified, UAHS RA will set the Summary Accrual field to YES on the PC Console > Main > Details.
- Studies using only questionnaires or surveys, including anonymous surveys.
- Studies collecting retrospective data.
- Studies collecting or using de-identified data or samples.
OnCore Support provides training and one-on-one assistance by email or via MS Bookings. OnCore Support can also attend departmental research coordinator meetings to provide information on OnCore features, workflows, and related Research Administration policies.
Guidelines and resources for research teams that are submitting the monthly Excel files are provided below:
Summary ACCRUAL TEMPLATE & example (oncology/non-oncology)
TicketCAt for upload of summary accrual
SUMMARY ACCRUAL TEMPLATE DEFINITIONS
OVERVIEW OF RELEVANT NIH, CCSG, AND HPP POLICIES
If you have any questions about this process, please reach out to COM IT by submitting a TicketCat request.
Minimum Data for Summary Accrual Studies
- Consent Date
- Institution
- Biological Sex
- Age Group
- Ethnicity
- Race
- Zip Code
- Number accrued
- Consent Date/On-Study Date
- Gender (Male, Female, Unknown, Undifferentiated, Unspecified)
- Race (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Other Pacific Islander, White, Unknown, Not Reported)
- Ethnicity (Hispanic or Latino, Non-Hispanic, Unknown, Not Reported)
- Age Group (0-9, 10-19, 20-29, 30-39, 40-49, 50-59, 60-69, 70-79, 80-89, 90-99, 100+, Unknown)
- Institution (University of Arizona Health Sciences (UAHS)) for most studies; other institutions may be selected such as UCLA, Yale, etc., but they must be listed in OnCore for the protocol)
- Disease Site/Diagnosis (Optional, will use Diagnosis for protocol)
- ZIP Code (Optional; if blank will use 99999)
- Recruited By (Optional)

